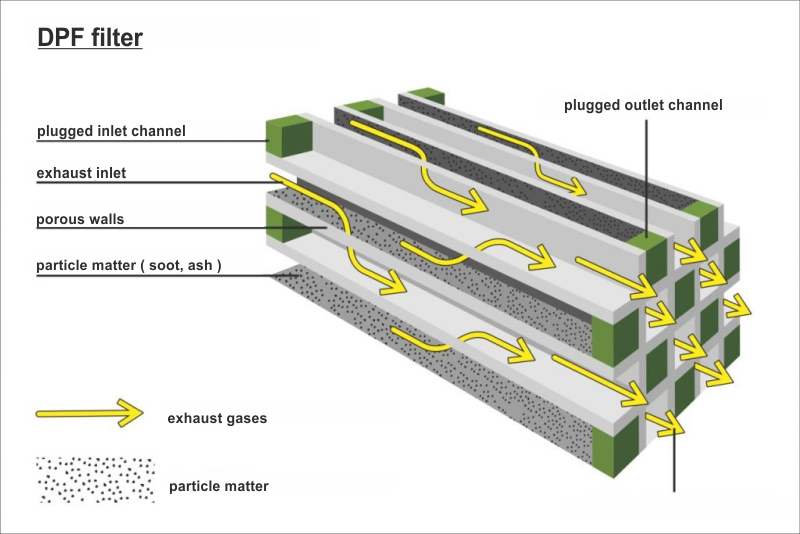
DPF structure
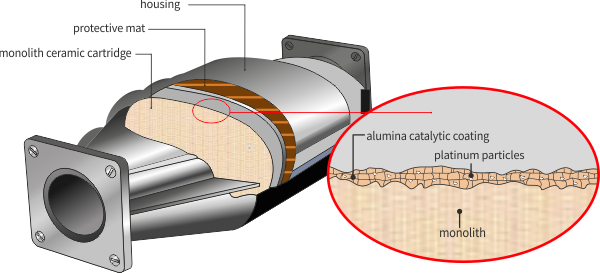 The Diesel Particulate Filter (DPF) removes particulates from Diesel engine exhaust through physical filtration. Many types of filters are available, but the most common is a ceramic monolith (cordierite or silicon carbide) with a honeycomb structure.
The Diesel Particulate Filter (DPF) removes particulates from Diesel engine exhaust through physical filtration. Many types of filters are available, but the most common is a ceramic monolith (cordierite or silicon carbide) with a honeycomb structure.
The particulate filter is similar to catalyst convertes (cross-section - honeycomb). However, the channels in the cartridge have a larger diameter and porous walls. In addition, they are coated with a catalytic coating that forms the foundation for the catalytic metal particles.
The channels plugged on the outlet side are called inlet ducts - the exhaust gases fall into the filter. In contrast, channels with plugged ends on the inlet side are outlet channels, where the exhaust gases escape.
The exhaust gases go through such an obstacle must squeeze through the porous walls leaving larger particles trapped inside the plugged channels.
Monoliths
They are made of cordierite, silicon carbide, or aluminum titanate.
The walls of the filter cartridge (monolith) have a structure of small size pores, which are carefully controlled in the production process. The total porosity of the material is usually between 45 and 50% or more, while the average pore sizes are typically between 10 and 20 μm. The filtration mechanism in monolithic wall filters is a combination of wall penetration and soot cake. Wall penetration is the dominant filtration mechanism in the first place because solid particles are deposited in the pore network inside the wall material. As the soot charge increases, the layer of particles forms along the wall surface in the inlet channels, and then soot cake filtration becomes the dominating mechanism. Typically, monolithic filters have a filtration efficiency between about 70 and 95% of all solid particles.
Catalytic coating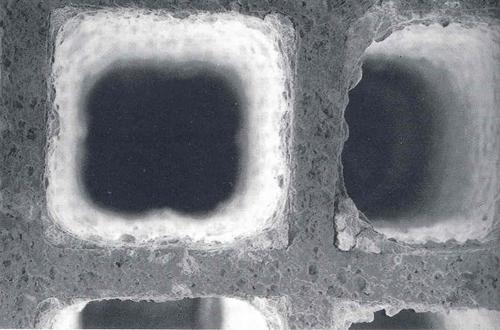
The main function of the catalytic coating is to provide a substrate for catalytic (noble) metals. In addition, the catalytic coating may physically separate and prevent undesirable reactions between components of the complex catalytic system.
Catalytic coating materials include inorganic non-noble metal oxides such as alumina, silicon oxide, cerium oxide, titanium dioxide, zirconium oxide, and zeolites.
Some of them are used as catalyst carriers, others are added to the catalytic coating as promoters or stabilizers, while others have catalytic activity.
Good catalytic coating materials are characterized by high thermal stability.
The catalytic coating is applied to a monolith with a water-based suspension.
Catalytic metals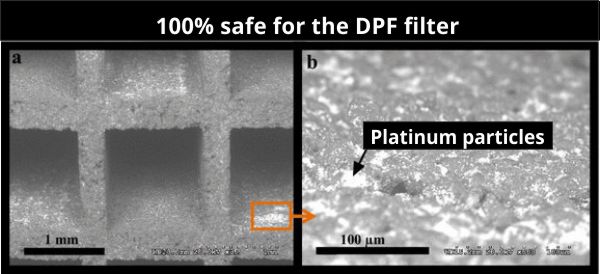
The noble metal catalyst (s) may be present in the catalytic slurry or they are used in a second step called impregnation. During impregnation, the catalytically coated monolith is immersed in an aqueous solution containing catalytic precursors. The catalyst is dried and calcined to its final form. During calcination, the catalyst precursors decompose to form a final catalyst, usually a metal or a metal oxide. The most common catalysts are platinum group (PGM) metals, such as platinum (Pt), palladium (Pd) and rhodium (Rh) alone.
Ceramic (protective) mat
It is wrapped around a monolith. Provides thermal insulation, protection against mechanical shocks and vibrations of the vehicle.